Summary: My wife had breast cancer. These posts describe: 1) finding out, 2) genetic testing, 3) radiation therapy, and 4) an incidental finding in the APC gene.

Incidental finding in the APC gene
Great news! Six months have passed since Kimberly finished radiation therapy for breast cancer. Today, she had a follow-up diagnostic mammogram that confirmed she is cancer-free! She will continue to be monitored over the next 5 years, but our big worries are behind us. Incidentally, we learned about a useful website during our journey, cancersurvivalrates.com that gave us a much better picture of survival rates.
Hereditary cancer screening
Let’s finish by returning to the variant in the APC gene that we found during expanded genetic testing and wrap-up this series.
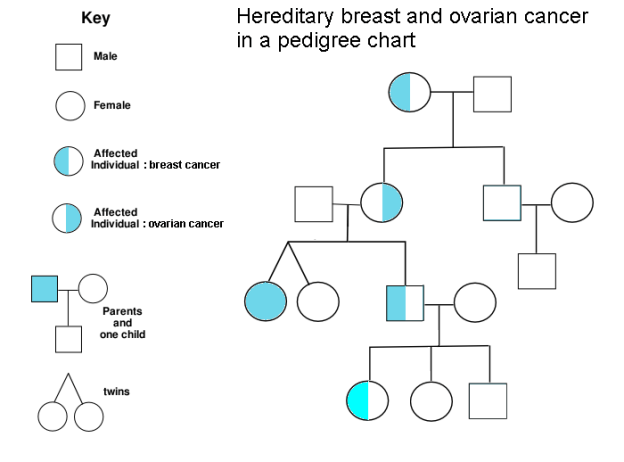
During genetic testing, our genetic counselor ordered an additional gene panel to screen for other cancers due to Kimberly’s family history. As I mentioned earlier, our insurance company denied all of our genetic testing claims, saying that the expanded panel was not related to her breast cancer. Nevertheless, the information that we received was worth the $250 out-of-pocket expense. Given the lack of reimbursement, reasonable costs for clinical genetic testing will ultimately drive most of it to be physician-ordered but privately paid. Just be sure to get your data!
So, what did we learn?
As we know from autosomal dominant inheritance, a person affected by an autosomal dominant disorder has a 50 percent chance of passing the mutated gene to each child. And sure enough, we saw the APC gene variant in 1 of our 2 adult-aged children; the other child does not carry it. We know this because we have whole genome sequences for everyone in our family. Here’s what Kimberly’s genetic code looks like at this location:

It turns out that this variant increases the risk of colorectal cancer from 5% (found in the general population) to 10% (in the population with this variant). So, the child with the variant should have a colonoscopy at age 40 (earlier than usual) and follow-up colonoscopies every 5 years after that. If you have a APC gene variant, talk to a genetic counselor–and show them some love! Note: This blog is not intended to replace advice from a medical professional.
Before publishing this story, we had a family meeting to discuss Mom’s cancer-free diagnosis, as well as the APC variant that one of them carries. All of us agreed to share this information with hopes that it will assist others.
Along the way, we learned that knowledge gave us the strength to move forward. I also have newfound appreciation for my wife, whose bravery knows no bounds.
/end